Content Row
Content Row

Tutorials & Extra Practice:
Ch 1: Matter and Measurement
Matter Vocab virtual flashcards
Scientific Notation ChemTour
Sig Fig ChemTour
Sig Fig practice virtual practice
Dimensional Analysis ChemTour
Volume conversions virtual practice
Mass conversions virtual practice
Distance conversions virtual practice
Density as a conversion virtual practice
Temperature Conversions ChemTour
Ch 2: Atoms, Molecules, and Ions
Cathode-Ray Tube Experiment ChemTour
Millikan Oil-Drop Experiment ChemTour
Rutherford Experiment ChemTour
Synthesis of Elements ChemTour
Atomic Theory Vocab virtual flashcards
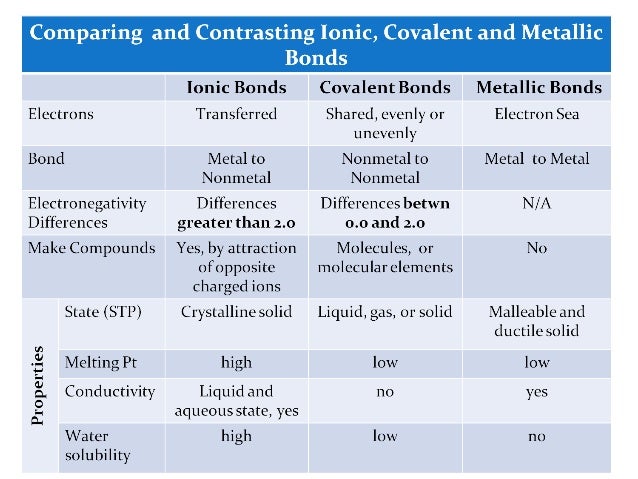
Naming ionic compounds virtual practice
Ionic compound formula writing virtual practice
Ch 3: Stoichiometry
Chemical Reactions Vocab virtual flashcards
Balancing Equations ChemTour
Balancing chemical equations practice virtual practice
NaCl Reaction ChemTour
Calculating formula weights and molar mass practice virtual practice
Percent Composition ChemTour
Calculating percent composition virtual practice
Avogradro's Number ChemTour
Mole Conversions (moles, mass, particles) virtual practice
STOICHIOMETRY virtual practice
Limiting Reactant ChemTour
Ch 4: Reactions in Aqueous Solution
Solution Vocab virtual flashcards
Molarity ChemTour
Dilutions ChemTour
Useful Video Lessons:

Bozeman Science: AP Chemistry
Aaron Sams & Jon Bergmann AP Chemistry
Hank Green Chemistry Crash Course
12/25/24 9:44 AM