
Tutorials & Extra Practice:
Ch 1: Matter and Measurement
- distinguish between atoms, elements, and compounds, ions, molecules, and mixtures
Matter Vocab virtual flashcards
- memorize the names and symbols of common elements
- recognize physical versus chemical changes and intensive versus extensive properties
- measure and calculate in metric units
Scientific Notation ChemTour
- distinguish between precision and accuracy
- measure and report calculated answers using significant figures
Sig Fig ChemTour
Sig Fig practice virtual practice
- problem solve using dimensional analysis
Dimensional Analysis ChemTour
Volume conversions virtual practice
Mass conversions virtual practice
Distance conversions virtual practice
Density as a conversion virtual practice
Temperature Conversions ChemTour
- calculate experimental error
- recognize lab safety rules
Ch 2: Atoms, Molecules, and Ions
- describe the structure of an atom
Cathode-Ray Tube Experiment ChemTour
Millikan Oil-Drop Experiment ChemTour
Rutherford Experiment ChemTour
Synthesis of Elements ChemTour
- describe the organization (properties and locations) of elements on the periodic table
- list the 7 diatomic elements (“HONClBrIF”)
- recognize vocabulary: cation, anion, chemical formula, structural formula
Atomic Theory Vocab virtual flashcards
- compare/ contrast ionic and molecular compounds
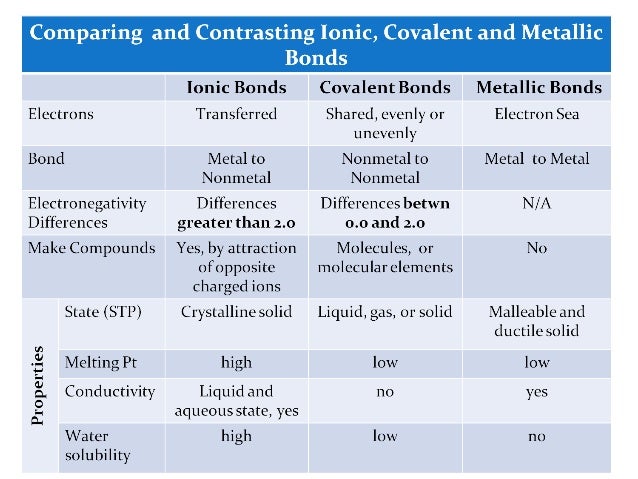
- memorize the names and charges of monatomic and polyatomic ions
- write formulas and name ionic and covalent (molecular) compounds
Naming ionic compounds virtual practice
Ionic compound formula writing virtual practice
- write and name simple organic compounds
Ch 3: Stoichiometry
- balance and interpret chemical reactions
Chemical Reactions Vocab virtual flashcards
Balancing Equations ChemTour
Balancing chemical equations practice virtual practice
NaCl Reaction ChemTour
- classify basic reaction types
- calculate formula weights/ molar masses
Calculating formula weights and molar mass practice virtual practice
- calculate percent composition
Percent Composition ChemTour
Calculating percent composition virtual practice
- recognize the MOLE as a chemical unit and Avogadro’s Number (6.02 x 1023 particles)
Avogradro's Number ChemTour
- convert between moles, mass, particles
Mole Conversions (moles, mass, particles) virtual practice
- calculate empirical and molecular formulas
- apply STOICHIOMETRY to analyze quantities of substances in a chemical reaction
STOICHIOMETRY virtual practice
- identify limiting and excess reactants and calculate theoretical and percent yields
Limiting Reactant ChemTour
Ch 4: Reactions in Aqueous Solution
- define solute, solvent, and solution
Solution Vocab virtual flashcards
- distinguish between the types of compounds that are electrolytes
- memorize the solubility rules
- predict the products of double replacement precipitation reactions
- distinguish between acids and bases
- memorize the 7 strong acids and 8 strong
- solve problems using Molarity as a unit of concentration
Molarity ChemTour
Dilutions ChemTour
Useful Video Lessons:

Bozeman Science: AP Chemistry
Aaron Sams & Jon Bergmann AP Chemistry
Hank Green Chemistry Crash Course